Types of Chromatography
Chromatography is a technique that uses the mobile phase, liquids, and gasses, to carry the components in a sample through the stationary phase to resolve it by sorption-desorption steps.
There are various types of chromatography processes, which include high-performance liquid chromatography (HPLC), gas chromatography (GS), and thin layer chromatography (TLC).
The High-Performance Liquid Chromatography (HPLC)
In high-performance liquid chromatography (HPLC), forms the differentiation and interaction of the associated two phases. These phases are the stationary phase and the mobile phase. HPLC is divided into various phases: chiral chromatography, ion exchange chromatography, normal phase chromatography, ion pair/affinity chromatography, and reverse phase chromatography (CDER, 1994).
- In chiral chromatography, enantiomers are separated by the formation of diastereomers on the chiral stationary phase. To achieve this kind of separation, mobile phase addictives or derivatizing agents are added in the phase. This technique is used in the impurity test method. The enantiomeric drug is separated from the enantiomeric impurity.
- Secondly, the ion exchange chromatography separates the components in the sample by using functional groups bearing the charges, for example, the cation exchange in the positive ion (X+) sample or anion exchange in the negative ion (X-) sample. In all the process, the PH is taken to be common (CDER, 1994).
- Thirdly, the ion-pair/affinity chromatography involves using a specific chemical reaction to separate the target species. The PH, type of organic solvents, the concentration, ionic strength, and temperature influence the separation of the sample involved. This separation is contributed by the addition of the buffer and counter-ion of opposite charge to the reverse phase mode. Affinity chromatography is mostly used in macromolecules where there is an interaction of the ligand with a homologous antigen. The buffer conditions can be altered to elude the reversible complex.
- The fourth stage involves the regular phase chromatography. This phase uses the polar stationary phase and the organic solvents, which act as a mobile phase. This system is based on the fact that the more polar components elute slower than the less polar components.
- Lastly, reverse phase chromatography, also known as the bonded phase chromatography, uses a simple mobile phase, the water. Majorly, the solvent strength influences the separation process. Other factors that also play a part in the separation process include the PH and the temperature. It is known that less polar components elute slowly compared to the more polar components and that all chromatography techniques use UV detection. These detectors provide the researcher with precise readings (CDER, 1994).
The Thin Layer Chromatography (TLC)
The second type of chromatography is the thin layer chromatography (TLC). This is the simplest form of chromatography and can be conducted manually. This process uses the coated plate, the stationary phase, and the mixture of solvents, which is the mobile phase. The migrating distinct components on the coated plate show the variations in the sample separation. The separated components are represented by the sample spots on the coated plate. Some compounds that have less coloration and detection techniques are used in the identification of the separated materials. These materials include the use of sprays, which are either universal or particular, UV, and fluorescence (CDER, 1994).
The Gas Chromatography (GC)
The third type of chromatography is the gas chromatography (GC). This technique uses gas to transport the volatilized sample through the stationary phase. In this case, gas is the mobile phase, which separates the samples through desorption and sorption. This process associates the chromatographic gas analysis. The study uses the gas, which is stable when subjected to a high temperature but volatile in nature. Another consideration is that these gasses should have low molecular weight as compared to other gasses. Most of the researchers use gas chromatography to separate drug products from residual drug solvents. Sometimes, the researchers can use chemical substances to add stability and volatility to the used gas. Detectors used in the process depend on the gasses used. For example, halogen compounds use electron capture (ECD); phosphorous and sulphur use flame detectors; phosphorous and nitrogen use nitrogen-phosphorous detectors (NPD); and carbon components are detected using a flame ionization detective (FID).
Method Performance
Method performance is the process of validating a chromatographic method used in the separation of the compound. Chromatography methods use the reference standards, which direct the processes in achieving a highly purified compound. The reference standard is based on the accurate information and quality. There are two types of reference standards, the USP/NF, which does not involve characterization, and the non-compendia standard, which characterizes the highest purity state to be achieved by a reasonable effort in the separation. It looks into deeper purification work to achieve a high quality of the products, identity, and strength.
There are two methods used by the chromatography test procedures in quantization analysis. They include the external analysis and the internal analysis.
- In the internal standard method, the quantization is assumed to be a result of the response of the target compound to internal standard over the response of the same preparation of the standard reference. The method is mainly based on HPLC and GC. Furthermore, the researchers find this method appropriate because of its complexity in the preparation procedures of the samples; the samples involved have minimum concentrations; and lastly, there is an expectation for the analysis of the broad range of concentrations.
- The external standard method is used in the analysis of the standard of using a separate chromatogram from the sample. Mostly, the quantitation of the analysis is based on the peak area spot intensity and height comparison of the tested samples. This method is used when the separated samples have a narrow range of concentration but a single target sample concentration. This is evident in the case release and acceptance tests. Secondly, extraneous peaks with potentiality can be detected easily because of the increased baseline. A good example is the impurities test. Lastly, the simplicity of preparation of the sample allows less time wastage and that the process can be understood and performed with minimal hardship (CDER, 1999).
Map Your Success Out!
Attract high grades like a magnet! Time to get on the right side of your professor.
When conducting the standard test, an effective concentration must be considered. It provides the concentration of the target compound. This process entails juxtaposing the sample standard and the concentration in line to improve the accuracy of the external method. Method performance is measured by ascertaining the HPL chromatographic validation parameters. These parameters include accuracy, linearity, quantization limit and detection limit, precision, range, recovery, robustness, stability in the sample solution, selectivity, system suitability, and tests.
For the accuracy to be determined in the experiment, the results must be relative to the real value. Accuracy is gained by the correct execution of the tests. The proper amount of drugs in consideration to weight and volume should be conducted. Linearity is also one of the important factors in the separation mechanism. Linearity must comply with the Obeys beer law, and that the linear detectability range must depend on the detectors used and the analyzed compound. Precision is an important factor in analyzing the effectiveness of the method used. It revolves around the repetitive execution of the separation processes. The data obtained from different methods must be closer to each other under the same conditions. This gives the precision of the method used. Robustness gives the process the ability to remain resilient to the external factors. This leads to relatively constant quantitative parameters. This shows the effectiveness of the method and its reliability. The range applied in the process must be relevant. It should be noted that the methods providing wide ranges are unreliable. Recovery is also affected by the preparation procedures of the samples. The simple preparation will provide lower recovery variations while the elaborate developments will give high recovery changes. Stability in the sample solution is conducted through evaluation of the drug products to the test method. This will indicate the suitability of the test method at hand. If the drug product is identified to be inappropriate, then the test method is unreliable. However, if the pharmaceutical product is defined to be appropriate, then the test method will be relevant.
The test sample must ensure that it has specificity. To obtain this, the test procedure should ensure that there is a minimal interference from extraneous components. These will lead to unspecific peaks due to the addition of known compounds. This will make the test methods unreliable. The suitability system specification is the general accuracy and the precision of the test method. System suitability and test are done by comparing all the parameters in the test analysis and summarizing them into the final report. Under this basis, the test method performance can be identified to be valid or null (CDER, 1999).
Chromatography Peak
Specific analyte band is detected to give the particular chromatography peak. Analyte bands are known to compose many analyte molecules, the center having the highest concentration. The sample interaction to the mobile phase leaves the concentration to be highest at the center and lower at the trailing edges. Detectors identify the separate dye bands as the bands move through the column. The detector measures the bands and relays proportional electrical signals to the computer. The computer will then record the data as peaks. Detectors have the ability to identify each color band from the mobile phase efficiently; they include UV, mass, and fluorescents.
Good peak shapes are determined by the Gaussian peak while the poor ones are identified by the peak fronting and tailing. The real peaks are known to possess narrow peak width and have the tailing factor of 1.0. These peaks have better efficiency. Their shapes also describe the peak. Best peaks are known to have improved resolutions and their quantization is more accurate. Peaks are separated based on their suitability, the column lifetime with the longer use.
Step 1. Order Placement
Step 2. Order Payment
Step 3. Paper Downloading
Peak Separation
Peak separation takes place as the band reaches the detector. The analyte interacts with the mobile phase in the chromatography system, which causes broadening of its width. This leads to formation of various brands known as the brand spreading. There is a direct relation between the analyte spread and the width of the chromatography peak. Outward widening of the analyte proportionally leads to an increase of the chromatographic pick. Likewise, minimized spread of the analyte will result in reduced width of the chromatographic peak. Different components in the samples have different spread in the stationary phase. These spread variations will automatically result in different chromatography peaks in the computer.
Different chromatography peaks will have distinct characteristics. They include: area of the peak, height of the peak, retention time of the peak, amount and symmetry of peak, width of the peak, the tailing of the peak, the resolution between the peaks, the capacity factor, the plate numbers, excess, skew, and relative selection to the successive peak. All these factors tend to give different peaks variety depending on the nature of the separated analytes. Symmetrical peaks are known to be good peaks while fronting and tailing peaks are considered to be poor peaks. To ascertain good peaks from poor peaks, measurements are conducted. They include USP tailing factor, efficiency, asymmetry, and peak width at half height.
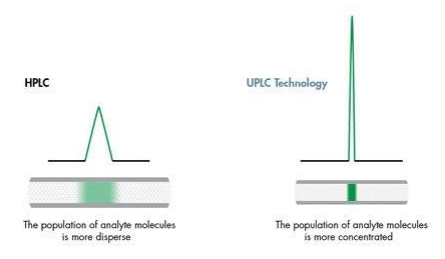
Figure 1: The effect of band spread on peak shape
Peak Interference
The resolution is the ability to separate peaks apart; when peaks are held together, they cannot be easily identified. This is one of the known forms of peaks interference; an individual is not able to distinguish the beginning of a separate peak and the ending of the other peak. These peaks are attached together, and they appear as one. These peaks are known to have poor resolution and hence termed as poor peaks. Resolutions are affected by various factors. In gas chromatography, an increase in the temperature or the carrier gas flow is known to be the major contributor to the poor resolution. This causes the tested sample, the vapor, to move fast through the column, leading to low retention time. Retention is known to allow time for the formation of the chromatography peaks; this is achieved through the interaction of the samples and the detectors. It should also be noted that the columns should be narrower and longer to provide better results. These two attributes allow for slow gas movement within the column. Another factor affecting the good resolution includes the solubility of the sample to the mobile phase. Good solubility in the mobile phase allows the sample composition to separate distinctively. The test should ensure that the sample chosen should match the mobile phase; for example, a non-polar sample, octane, should be used with its non-polar counterpart, silicon oil. If the test is changed in using the polar samples, alcohol, the same polar mobile phase should be used, the polythene glycol (Matt, 2011).
Tails in the Peaks
Peaks tails exist in two forms, the early eluting peak tails and the later eluting peak tails. The two peak tails happen in different ways. Early peak tails occur when performing volatile analysis using the headspace, trap, purge, and even the sample loop for the gas sample. This problem is caused by the leaks in the test or the low volume. This is experienced on the analytical tube’s upstream. Other factors are also known to create this form of a problem; they include a substance stuck in the column, which has the potentiality of preventing the eluting mechanism. There are also minor errors contributing to the eluting processes, a poorly installed analytical column or poor cut allow for the sample escape from the sample column. The volatile substances escape faster into the air as compared to the less volatile substances. It should be known that, in order to avoid the tail peaks, the sample vanishing points must be checked (Alan, 2013).
In semi-volatile materials, when using solvent-volatile analysis, the temperature of 20-40 degrees Celsius is recommended to avoid tail peaks. This is necessitated to allow the compounds and the associated solvent to condense inside the analytical column. An increase in the temperature will cause the “solvent effect violation”, which is associated with awkward compound peaks, which are rounded, tailed, and broad. Lower temperatures are majorly used to orient the compound peaks (Alan, 2013).
In the later eluting peaks, tail is caused by the column contamination, low rates of gas flow, and the cold spots in the analytical column. Low ion source temperature may be the cause of tailed peaks if spec mass is used. In some instances, the column lines may be contaminated or oxidized, leading to tail peaks. To avoid this issue from interfering with the system, all proper zones must be heated in the column; the instrument must be baked to remove the contamination in the column. There should be a removal of the transfer line section involving the flow rate of the carrier gas.
The inefficiency of the system may cause a tail peak. This entails the peaks that are seen in the entire chromatogram tail. The system may adopt lack of sensitivity in identifying certain compounds in the sample.
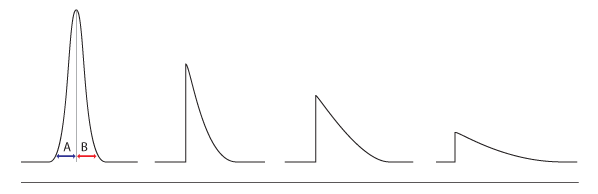
Figure 2. Peak tails
Reversed Phase in HPLC
Reversed phase in HPLC is the chromatography separation technique that depends on the binding association of the solute molecule in the mobile phase and immobilized hydrophobic ligand. The entropy effect leads to this binding relation. In this association, the solutes are exposed to the immobilized hydrophobic ligand and get stuck. This leads to the minimized hydrophobic region exposed to the solvent. The increase in the systems entropy minimizes the organized water structure. The reverse chromatography uses the adsorptive process, which relies on the partitioning mechanism to separate the components in the sample. The use of silica as the base matrix is the major problem with the reverse method. Silica possesses a high form of chemical instability. Silica is associated with dissolution when subjected to high PH; moreover, silica is not efficient under the PH of 7.5 when in aqueous solutions. The solution to this problem is the use of synthetic organic polymers, which are stable under all PH and have excellent chemical stability. The PH stability ranges between 1-12. If we consider silica gel, the separation activity cannot be carried out at PH above 7.5.
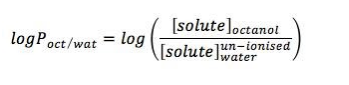
Figure 3. Increased hydrophobic molecules are influenced by higher value of log P (between -1 and +1).
Peak Purity
The better part of sample separation is maintaining the purity to ensure that added impurities do not interfere with the peaks response. The test must include impurity test before the qualitative information from the electrophoretic peak is used in the calculations. Impurities may interfere with the final results, leading to information loss (Stahl, 2003). Peak purity can be analyzed by looking into various criteria: the comparison in peak spectra, the similarity factors, sensitivity improvement and reliability, similarity and threshold (without any transformation or as a natural logarithm), similarity threshold ratio, and the purity ratio.
Comparison of peak spectra involves comparing different peak spectra in the analysis. Greater deviation from each other shows the presence of the impurity. Displaying values achieves purity ratio for each spectrum. These values are the logarithm of the threshold difference of each spectrum (Stahl, 2003). The similarity and threshold, those without any transformation, entail the displayed curves, which are calculated using values ranging between 1 and 1,000. The similarity and threshold in the natural logarithm show the natural logarithm of the calculated value (Stahl, 2003).
Obtention Separation (Resolution)
Resolution is the ability of the peak to be clearly identified as being different. The proper resolution must have average width height. Very low height shows the poor resolution and the same to the above required height standards. To achieve the acceptable medium height, the attenuation must be conducted. A good value resolution is represented when the peaks have minor tailing. A value of Rs is 2.0. This give the adequate value; it should be known that the increase in tailing activates the requirement for resolution because the higher the tail, the lower the resolution (Dolan, 2015).
Retention Factor
Retention factor is the ratio of the distance spot moved over the distance solvent traveled. The retention value is calculated to determine the travel of each component. The usefulness of the retention factor is seen where the results of one chromatogram can be compared to the results of another chromatogram. Comparison of the chromatogram results should be constant. Retention factor having the constant value allows the unknown to be matched with the known materials. A critical use is when the unknown presents the different value far away from the known then the materials are not of the same material. It should be known that the retention factor is not the same from sample to sample and that variation can be experienced (Harper College).
Don't spend another minute
worrying about deadlines or grades. Have a question? Chat with us!
